BIO InvestorForum 2007
Ms. Mary Anne Dillahunty
Vice President, Intellectual Property
Oncolytics Biotech Inc.
October 11, 2007.

(Slide 1)Thank you. Good afternoon. Thank you for coming.
 (Slide 2) I would like to draw your attention first of all to these forward-looking statements. We are a public company, so we are required to say this.
(Slide 2) I would like to draw your attention first of all to these forward-looking statements. We are a public company, so we are required to say this. (Slide 3) Okay, Oncolytics' lead product is Reolysin which is a targeted anticancer therapy. It is a therapy based on a reovirus. And we have a broad-based phase II program, clinical trial program that is ongoing in the US and in the UK. In the clinical trials that we have done and we are doing, Reolysin has an excellent safety profile and its mechanism of action is actually directly cytotoxic in that we have seen tumour responses in a variety of cancers. With just single therapy with Reolysin itself, without any co-therapy. In addition, we have found that there are synergistic effects with the common chemotherapeutics and the conventional chemotherapies, and also with radiotherapy. Reolysin potentiates most cytotoxics that it has been tried in combination with. And we are now exploring that further with three combination trials that are enrolling patients.
(Slide 3) Okay, Oncolytics' lead product is Reolysin which is a targeted anticancer therapy. It is a therapy based on a reovirus. And we have a broad-based phase II program, clinical trial program that is ongoing in the US and in the UK. In the clinical trials that we have done and we are doing, Reolysin has an excellent safety profile and its mechanism of action is actually directly cytotoxic in that we have seen tumour responses in a variety of cancers. With just single therapy with Reolysin itself, without any co-therapy. In addition, we have found that there are synergistic effects with the common chemotherapeutics and the conventional chemotherapies, and also with radiotherapy. Reolysin potentiates most cytotoxics that it has been tried in combination with. And we are now exploring that further with three combination trials that are enrolling patients.And addition it has been shown to synergistically potentiate radiotherapy, and we are also enrolling patients in a phase II combination radiotherapy and local Reolysin program.
 (Slide 4) So, this slide just gives an overview of what I plan to talk about today. I'll tell you a little bit about the mode of action of Reolysin, something about our intellectual property portfolio, our manufacturing, and the safety of Reolysin, and then go into a little bit of detail on our clinical trial program.
(Slide 4) So, this slide just gives an overview of what I plan to talk about today. I'll tell you a little bit about the mode of action of Reolysin, something about our intellectual property portfolio, our manufacturing, and the safety of Reolysin, and then go into a little bit of detail on our clinical trial program. (Slide 5) Oncolytics Biotech Inc. is a Calgary Alberta Canada based company whose corporate focus is on the development of Oncolytic viruses for the treatment of cancer. We have what we think is a comprehensive and ongoing clinical program with our lead product Reolysin. And in addition to having the program planned, we have the funding in place to support the phase II clinical trials that we have planned.
(Slide 5) Oncolytics Biotech Inc. is a Calgary Alberta Canada based company whose corporate focus is on the development of Oncolytic viruses for the treatment of cancer. We have what we think is a comprehensive and ongoing clinical program with our lead product Reolysin. And in addition to having the program planned, we have the funding in place to support the phase II clinical trials that we have planned.Again our lead product is Reolysin.
 (Slide 6) This slide and on the left panel of this side you can see, the sort of schematic of how reovirus and Reolysin lyse cancer cells. Reolysin contains naturally occurring reovirus. This is replication competent virus; it's not genetically modified to carry any heterologous genes. Reovirus itself is asymptomatic in humans, although many people have been exposed to reovirus it doesn't cause disease generally, and part of the reason is that reovirus selectively replicates only in RAS activated cells. And it turns out that RAS activated cancers comprise about two thirds of all cancers, so it is very broad-based. We have estimated that at least 5 million new patients a year are going to develop cancers with RAS involvement and therefore would be targets for Reolysin therapy.
(Slide 6) This slide and on the left panel of this side you can see, the sort of schematic of how reovirus and Reolysin lyse cancer cells. Reolysin contains naturally occurring reovirus. This is replication competent virus; it's not genetically modified to carry any heterologous genes. Reovirus itself is asymptomatic in humans, although many people have been exposed to reovirus it doesn't cause disease generally, and part of the reason is that reovirus selectively replicates only in RAS activated cells. And it turns out that RAS activated cancers comprise about two thirds of all cancers, so it is very broad-based. We have estimated that at least 5 million new patients a year are going to develop cancers with RAS involvement and therefore would be targets for Reolysin therapy. (Slide 7) This is sort of a quick and I have a couple of slides here to show you what happens when reovirus invades a non-RAS active cell vs. a RAS activated cell. Reovirus actually infects both normal and RAS active cells it is just unable to reproduce, to replicate in normal cells, such as this non-RAS active cell which schematically presented here. When RAS is inactive the active PKR prevents translation of the viral mRNA and the virus can't replicate.
(Slide 7) This is sort of a quick and I have a couple of slides here to show you what happens when reovirus invades a non-RAS active cell vs. a RAS activated cell. Reovirus actually infects both normal and RAS active cells it is just unable to reproduce, to replicate in normal cells, such as this non-RAS active cell which schematically presented here. When RAS is inactive the active PKR prevents translation of the viral mRNA and the virus can't replicate. (Slide 8) Whereas in a RAS activated cell. The RAS blocks PKR and the virus is able to replicate.
(Slide 8) Whereas in a RAS activated cell. The RAS blocks PKR and the virus is able to replicate.So that's the selective mode of action of Reovirus and Reolysin.
 (Slide 9) Now, this slide is a depiction of the two modes of action that we believe Reolysin uses to inhibit tumour growth and regress tumours. Primary mode of action of course is that the reovirus selectively invades tumour cells, replicates, makes more virus particles, which go out and invade more tumour cells and cause lyses of the tumour. In addition, we believe and we have some evidence that I'll discuss a little later that would to lead us to believe that when these tumour cells lyse the tumour antigens are released and the immune system of the patient then is able to form antibodies against the tumour antigens. So that's thesecondary mode
(Slide 9) Now, this slide is a depiction of the two modes of action that we believe Reolysin uses to inhibit tumour growth and regress tumours. Primary mode of action of course is that the reovirus selectively invades tumour cells, replicates, makes more virus particles, which go out and invade more tumour cells and cause lyses of the tumour. In addition, we believe and we have some evidence that I'll discuss a little later that would to lead us to believe that when these tumour cells lyse the tumour antigens are released and the immune system of the patient then is able to form antibodies against the tumour antigens. So that's thesecondary modeof killing the tumour cells.
 (Slide 10)Oncolytics Biotech has a very large intellectual property portfolio. We have over 150 issued patents worldwide. That includes a couple dozen in the US, six in Canada, we have issued patents in Europe, allowed claims in Japan. And with respect to our Reovirus patent portfolio those claims cover composition of matter, both of reovirus and reovirus in combination with other treatments, and also methods of treatments and methods of manufacturer.
(Slide 10)Oncolytics Biotech has a very large intellectual property portfolio. We have over 150 issued patents worldwide. That includes a couple dozen in the US, six in Canada, we have issued patents in Europe, allowed claims in Japan. And with respect to our Reovirus patent portfolio those claims cover composition of matter, both of reovirus and reovirus in combination with other treatments, and also methods of treatments and methods of manufacturer.In addition to our Reovirus IP portfolio, we have patents to other viruses which are oncolytic to RAS activated tumour. And we have issued patents directed to adenovirus and herpes virus therapies as well. These viruses are genetically engineered.
We have in addition to our 150 issued patents, we have more than 180 pending patent applications worldwide, and those patterns are in 30 families, very broad, and we continue to file new patent applications that cover our commercial products.
 (Slide 11) Manufacturing of Reolysin was we believe a key factor in the success of the product and we are very proud of the success we've had there. Its obviously critical to be able to have products to use in our clinical trials and commercially and we have been able to successfully develop proprietary cell growth medium, and we have GNP material produced at the 20 liter scale and we just completed a 40 liter scale up which provided more than 20,000 doses of Reolysin at three 3x10 to the 10th TCID50 and that's per 40 liter run. So that's pretty good, we are at the hundred liter scale up work right now and we are comfortably able to provide virus for all of our ongoing and planned phase II clinical trials.
(Slide 11) Manufacturing of Reolysin was we believe a key factor in the success of the product and we are very proud of the success we've had there. Its obviously critical to be able to have products to use in our clinical trials and commercially and we have been able to successfully develop proprietary cell growth medium, and we have GNP material produced at the 20 liter scale and we just completed a 40 liter scale up which provided more than 20,000 doses of Reolysin at three 3x10 to the 10th TCID50 and that's per 40 liter run. So that's pretty good, we are at the hundred liter scale up work right now and we are comfortably able to provide virus for all of our ongoing and planned phase II clinical trials.To give you an idea, I've been told that in the picture that's 9 L of the virus and if we were selling the virus at what we anticipate we will be selling it at, that's $50 to $100 million worth of virus in that glass.
 (Slide 12) Reolysin as far as we know in all the studies that we have done, with over 130 patients, has a very good safety profile. These patients have received Reolysin either intratumourally, intracerebrally, or systemically IV at a doses of high as 3x10 to the 10th. And we have not reached the maximum tolerated dose to date. The toxicities that we have seen have generally been mild. The patients on our studies have described them by and large as pre-flu like symptoms. What it feel like when you are about to come down with the flu. They are reversible and we've been able to manage them with something like a Tylenol.
(Slide 12) Reolysin as far as we know in all the studies that we have done, with over 130 patients, has a very good safety profile. These patients have received Reolysin either intratumourally, intracerebrally, or systemically IV at a doses of high as 3x10 to the 10th. And we have not reached the maximum tolerated dose to date. The toxicities that we have seen have generally been mild. The patients on our studies have described them by and large as pre-flu like symptoms. What it feel like when you are about to come down with the flu. They are reversible and we've been able to manage them with something like a Tylenol. (Slide 13) This slide, which is a very busy slide, gives an overview of our clinical trial program, as of right now you can see that it's in four separate areas and I will be describing each of those as we go on.
(Slide 13) This slide, which is a very busy slide, gives an overview of our clinical trial program, as of right now you can see that it's in four separate areas and I will be describing each of those as we go on. (Slide 14) Okay, so there are really two branches in our clinical trial program. One is monotherapy and that is treating cancers with single agent, mainly Reolysin, without any co-therapies and the other arm is co-therapy.
(Slide 14) Okay, so there are really two branches in our clinical trial program. One is monotherapy and that is treating cancers with single agent, mainly Reolysin, without any co-therapies and the other arm is co-therapy.In the single agent activity, in the monotherapy, again there are two branches: local delivery, intratumourally; and then systemic delivery, IV. The cotherapy which is what we believe is probably the anticipated way we are going to get to commercial use has again a local and systemic aspect to it. The local delivery being used intratumourally in conjunction with radiotherapy, and systemic delivery with systemic chemotherapy.
 (Slide 15) Just to give you an idea, I know you probably can't read all the places on this map. But this is a map to show you how extensive our clinical trial sites are. They are all over the world: Asia, Europe, and the U.S. The orange dots are the sites that Oncolytics has trials going and the yellow dots are sites where we are plan to work with NCI on. So you can see that we have extensive clinical trials throughout the world.
(Slide 15) Just to give you an idea, I know you probably can't read all the places on this map. But this is a map to show you how extensive our clinical trial sites are. They are all over the world: Asia, Europe, and the U.S. The orange dots are the sites that Oncolytics has trials going and the yellow dots are sites where we are plan to work with NCI on. So you can see that we have extensive clinical trials throughout the world. (Slide 16) I'm going to tell you a little bit more about some of those. We have some results from a phase I/II intratumoural recurrent malignant glioma study that is currently enrolling in the phase I portion. This is a single product infusion, in other words, a single dose of Reolysin in patients with recurrent malignant glioma. This is a dose escalation study and then we expect that as we reach the MTD there will be a treatment arm. The product Reolysin is infusion delivered. It's not local, it’s systemic, and we have done three previous studies, phase I glioma studies, and one of those studies we have had 12 patients and six of those 12 patients actually lived more than six months, three of them lived longer than a year, and one patient (we believe) as far as we know is still alive. And certainly we know that the patient has survived longer than five years, so we were very heartened by those results.
(Slide 16) I'm going to tell you a little bit more about some of those. We have some results from a phase I/II intratumoural recurrent malignant glioma study that is currently enrolling in the phase I portion. This is a single product infusion, in other words, a single dose of Reolysin in patients with recurrent malignant glioma. This is a dose escalation study and then we expect that as we reach the MTD there will be a treatment arm. The product Reolysin is infusion delivered. It's not local, it’s systemic, and we have done three previous studies, phase I glioma studies, and one of those studies we have had 12 patients and six of those 12 patients actually lived more than six months, three of them lived longer than a year, and one patient (we believe) as far as we know is still alive. And certainly we know that the patient has survived longer than five years, so we were very heartened by those results. (Slide 17) Another study that we are looking into is the phase IIa combination of Reolysin and radiotherapy. And this study is also going to focus on patients that have head/neck/oesophageal cancer. This will involve intratumoural administration of Reolysin with 20 Gy radiation and this will be in patients with advanced or metastatic solid tumours refractory to standard therapy. So we started enrollment in December of last year of the phase IIa study and the study is expected to be 40 patients with half of them in the head/neck/oesophageal cancer area. Part of the reason we designed the study the way did is that in preclinical studies we saw that there was a synergistic effect of radiation and Reolysin and we also had some clinical results in a phase Ia/Ib study that we did in the UK we were very excited, because even though we had local dosing of Reolysin, and of course we got local responses, we also got some remote responses, and the next couple slides will discuss that.
(Slide 17) Another study that we are looking into is the phase IIa combination of Reolysin and radiotherapy. And this study is also going to focus on patients that have head/neck/oesophageal cancer. This will involve intratumoural administration of Reolysin with 20 Gy radiation and this will be in patients with advanced or metastatic solid tumours refractory to standard therapy. So we started enrollment in December of last year of the phase IIa study and the study is expected to be 40 patients with half of them in the head/neck/oesophageal cancer area. Part of the reason we designed the study the way did is that in preclinical studies we saw that there was a synergistic effect of radiation and Reolysin and we also had some clinical results in a phase Ia/Ib study that we did in the UK we were very excited, because even though we had local dosing of Reolysin, and of course we got local responses, we also got some remote responses, and the next couple slides will discuss that. (Slide 18) This slide has three panels. It shows a patient who had supraclavicular lymph node cancer. This panel on the left is at the time of treatment. And you can see the tumour, which is shown in green, was quite enlarged, has moved to the esophagus, which is the dark space, aside. The patient was having trouble swallowing, etc. This middle panel is actually two months after the single course of treatment with intratumoural Reolysin and radiation. And by the way 20 Gy radiation is not curative but palliative, so you can see there was tumour regression and the esophagus was moving back into place. Most remarkably to our mind was the far right panel, which was taken seven months after the single course of treatment ,and you can see that tumour regression continues to occur and so there was clearly continued activity after there was no longer any reovirus in the body.
(Slide 18) This slide has three panels. It shows a patient who had supraclavicular lymph node cancer. This panel on the left is at the time of treatment. And you can see the tumour, which is shown in green, was quite enlarged, has moved to the esophagus, which is the dark space, aside. The patient was having trouble swallowing, etc. This middle panel is actually two months after the single course of treatment with intratumoural Reolysin and radiation. And by the way 20 Gy radiation is not curative but palliative, so you can see there was tumour regression and the esophagus was moving back into place. Most remarkably to our mind was the far right panel, which was taken seven months after the single course of treatment ,and you can see that tumour regression continues to occur and so there was clearly continued activity after there was no longer any reovirus in the body. (Slide 19) And very excitingly, this is the same patient from the previous slide; we also saw a response outside the radiation field. This was a metastatic node in the same patient the panel of on the left is at the time a treatment panel the right to seven months later. So clearly either the reovirus was able to systemically be delivered to a remote cancer, this metastatic node and lyse the cancel cells there, or the secondary mode of action that I showed on the slide where the tumour antigens stimulated the body to mount an immune response against tumour antigens wherever they were found happened. So we got some remote response.
(Slide 19) And very excitingly, this is the same patient from the previous slide; we also saw a response outside the radiation field. This was a metastatic node in the same patient the panel of on the left is at the time a treatment panel the right to seven months later. So clearly either the reovirus was able to systemically be delivered to a remote cancer, this metastatic node and lyse the cancel cells there, or the secondary mode of action that I showed on the slide where the tumour antigens stimulated the body to mount an immune response against tumour antigens wherever they were found happened. So we got some remote response. (Slide 20) Now systemic administration is really what we believe is going to be the largest potential patient population for Reolysin, and so many of our clinical trials are using systemic administration both in monotherapy and in combination therapy. So far in over 50 patients, systemic administration of Reolysin has been very well tolerated and we have two completed phase I studies, and that was found. In addition, we have had demonstrable tumour regression in a number of different cancer indications. So it's been very promising.
(Slide 20) Now systemic administration is really what we believe is going to be the largest potential patient population for Reolysin, and so many of our clinical trials are using systemic administration both in monotherapy and in combination therapy. So far in over 50 patients, systemic administration of Reolysin has been very well tolerated and we have two completed phase I studies, and that was found. In addition, we have had demonstrable tumour regression in a number of different cancer indications. So it's been very promising. (Slide 21) We have some results from our phase I monotherapy with Reolysin can only systemic administration trials for both the UK and the US. In the UK we had the reovirus that was delivered IV. And was able to infect, stabilize, or regress tumours in a variety of different locations and tumour types. And in addition, we took tumour biopsies in some of the patients, and we were able to isolate live virus from the biopsy showing that systemic administration was able to deliver a virus to the tumour and the virus is able to replicate there.
(Slide 21) We have some results from our phase I monotherapy with Reolysin can only systemic administration trials for both the UK and the US. In the UK we had the reovirus that was delivered IV. And was able to infect, stabilize, or regress tumours in a variety of different locations and tumour types. And in addition, we took tumour biopsies in some of the patients, and we were able to isolate live virus from the biopsy showing that systemic administration was able to deliver a virus to the tumour and the virus is able to replicate there.In the phase I US results, we had 18 patients treated. And 8 of those 18 of those patients demonstrated stable disease or better including one partial response in a breast cancer patient. Again the toxicities related to treatment were very mild and very manageable.
 (Slide 22) So this is a scan a CT scan from a patient in the UK study: Metastatic Prostate Cancer. You can see the two arrows in the left panel are taken pretreatment show enlarged lymph nodes to the left and to right of the bladder. You can see the color is very uniform. In contrast, if you look on the right side of the panel, if you look in the two arrows, there in the area of the lymph nodes (while there is some regression in size) you can see that the colors shows, there is necrosis of the tumour tissue. In addition to, and not shown on this slide, the PSA level in this patient went from about 100 pretreatment to 50 after Reolysin treatment.
(Slide 22) So this is a scan a CT scan from a patient in the UK study: Metastatic Prostate Cancer. You can see the two arrows in the left panel are taken pretreatment show enlarged lymph nodes to the left and to right of the bladder. You can see the color is very uniform. In contrast, if you look on the right side of the panel, if you look in the two arrows, there in the area of the lymph nodes (while there is some regression in size) you can see that the colors shows, there is necrosis of the tumour tissue. In addition to, and not shown on this slide, the PSA level in this patient went from about 100 pretreatment to 50 after Reolysin treatment. (Slide 23) This is an electron micrograph of a tissue biopsy from the same patient and it's hard to tell because it's so close in, but there was widespread necrosis of the tumour tissue. So there was tumour cell death after Reolysin treatment systemically. In addition you can see by the arrows and the circled area that the virus is able to deliver to and infect the tumour cells and also that there was factory formation which is indicated by the circles. So the virus was able to not just infect him but replicate. As I said we did tumour biopsies and we were able to isolate up to 10 to the 7th infectious virus particles per gram of tumour tissue after the systemic administration.
(Slide 23) This is an electron micrograph of a tissue biopsy from the same patient and it's hard to tell because it's so close in, but there was widespread necrosis of the tumour tissue. So there was tumour cell death after Reolysin treatment systemically. In addition you can see by the arrows and the circled area that the virus is able to deliver to and infect the tumour cells and also that there was factory formation which is indicated by the circles. So the virus was able to not just infect him but replicate. As I said we did tumour biopsies and we were able to isolate up to 10 to the 7th infectious virus particles per gram of tumour tissue after the systemic administration. (Slide 24) So, we are enrolling patients right now in a phase II sarcoma study. We are going to be treating those patients' IV with 3x10 to the 10th TCID50 for five days with re-treatment on a monthly basis. Enrollment has started. In addition, we have a couple other phase I/II monotherapy studies planned or in various stages. The phase II the melanoma study which will be done in conjunction with NCI should enroll 50 patients, and they'll have received Reolysin IV the protocol has to be submitted to the FDA for this study.
(Slide 24) So, we are enrolling patients right now in a phase II sarcoma study. We are going to be treating those patients' IV with 3x10 to the 10th TCID50 for five days with re-treatment on a monthly basis. Enrollment has started. In addition, we have a couple other phase I/II monotherapy studies planned or in various stages. The phase II the melanoma study which will be done in conjunction with NCI should enroll 50 patients, and they'll have received Reolysin IV the protocol has to be submitted to the FDA for this study.The phase I/II ovarian cancer clinical trial has been planned will enroll approximately 64 patients. This again will be done in conjunction with NCI and those patients will receive Reolysin both IV and IP.
 (Slide 25) So right now we are also enrolling patients in three phase II systemic administration studies where Reolysin is being administered in combination with standard chemotherapeutics. And right now patients are being enrolled and you can see that we are using Reolysin in conjunction with docetaxel, paclitaxel, carboplatin, and gemcitabine. These drugs which are actually manufactured and sold by different companies have combined or have and had combined peak sales of about $7 billion per year. So we believe that if we can develop Reolysin and show how efficacious it is, how synergistic it is with these products. We'll really have a large market potential for Reolysin.
(Slide 25) So right now we are also enrolling patients in three phase II systemic administration studies where Reolysin is being administered in combination with standard chemotherapeutics. And right now patients are being enrolled and you can see that we are using Reolysin in conjunction with docetaxel, paclitaxel, carboplatin, and gemcitabine. These drugs which are actually manufactured and sold by different companies have combined or have and had combined peak sales of about $7 billion per year. So we believe that if we can develop Reolysin and show how efficacious it is, how synergistic it is with these products. We'll really have a large market potential for Reolysin. (Slide 26) Now part of the reason, and part of the rationale the kind of study design of these combination clinical studies was because we had evidence from preclinical studies that Reolysin was synergistic with a variety of different chemotherapeutic agents and a variety of cancers. This slide shows just how many chemotherapeutic has been tried with and been shown to be synergistic. You can see there is quite a range. Cisplatin, 5-FU, doxorubicin, docetaxel, and others. And this work has been done by collaborators all over the world with the same results. And the list of collaborators is also on this slide. And I want to point out that of course is synergistic with the common chemotherapeutic. But in addition, we have also shown that it's efficacious as a monotherapy all by itself.
(Slide 26) Now part of the reason, and part of the rationale the kind of study design of these combination clinical studies was because we had evidence from preclinical studies that Reolysin was synergistic with a variety of different chemotherapeutic agents and a variety of cancers. This slide shows just how many chemotherapeutic has been tried with and been shown to be synergistic. You can see there is quite a range. Cisplatin, 5-FU, doxorubicin, docetaxel, and others. And this work has been done by collaborators all over the world with the same results. And the list of collaborators is also on this slide. And I want to point out that of course is synergistic with the common chemotherapeutic. But in addition, we have also shown that it's efficacious as a monotherapy all by itself.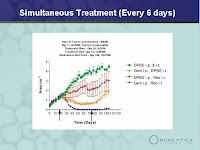 (Slide 27) This slide presents some animal preclinical data that we're very excited about which is also a combination therapy. This slide, the study was done in mice with a human colon cancer cell line. The Green line on the top shows the tumour grows without any treatment, and then the red line shows treatments with Reolysin on its own. The Orange line is treatment with gemcitabine and the blue line down on the bottom is combined treatments with both Reolysin and gemcitabine and as you can see the tumour was completely cured...I'd hate to...you know...that's dicey to say, but we believe it really was, not just inhibited. But if you look at the right-hand arrows, the left hand arrow is the beginning of treatment, the right-hand arrows is the end of treatment and you can see that even days and days and days after treatment ended with these two agents there was no recurrence of tumours. This is very excited were looking forward to seeing the clinical trial results.
(Slide 27) This slide presents some animal preclinical data that we're very excited about which is also a combination therapy. This slide, the study was done in mice with a human colon cancer cell line. The Green line on the top shows the tumour grows without any treatment, and then the red line shows treatments with Reolysin on its own. The Orange line is treatment with gemcitabine and the blue line down on the bottom is combined treatments with both Reolysin and gemcitabine and as you can see the tumour was completely cured...I'd hate to...you know...that's dicey to say, but we believe it really was, not just inhibited. But if you look at the right-hand arrows, the left hand arrow is the beginning of treatment, the right-hand arrows is the end of treatment and you can see that even days and days and days after treatment ended with these two agents there was no recurrence of tumours. This is very excited were looking forward to seeing the clinical trial results. (Slide 28) This slide is just a general overview of our market and capital data. We are public company traded on both NASDAQ and the Toronto Stock exchange we have as of June 30th, 41 and 49-1/2 (approximately) million shares outstanding. As of June 30th we had 31-1/2 million dollars Canadian with a monthly burn rate of 1.4 million and that gives us cash well into 2009.
(Slide 28) This slide is just a general overview of our market and capital data. We are public company traded on both NASDAQ and the Toronto Stock exchange we have as of June 30th, 41 and 49-1/2 (approximately) million shares outstanding. As of June 30th we had 31-1/2 million dollars Canadian with a monthly burn rate of 1.4 million and that gives us cash well into 2009. So in summary, we have found that Reolysin is a very broadly active novel cancer therapy, and we have engaged in and are continuing to progress through a focused clinical program. We have finished six clinical trials and have seven ongoing. And we expect that the enrollment will conclude in several of the phase II studies next year. And all of the interim and final data we have show that there are very positive results. We have a growing intellectual property portfolio with broad patent coverage all over the world, and especially in US, Europe and Canada. Solid balance sheets with enough funding to get us through our planned phase II clinical program. And we able to manufacture a product at commercial scale. So. Thank you very much, if anybody has any questions I will be upfront. Thank you.
So in summary, we have found that Reolysin is a very broadly active novel cancer therapy, and we have engaged in and are continuing to progress through a focused clinical program. We have finished six clinical trials and have seven ongoing. And we expect that the enrollment will conclude in several of the phase II studies next year. And all of the interim and final data we have show that there are very positive results. We have a growing intellectual property portfolio with broad patent coverage all over the world, and especially in US, Europe and Canada. Solid balance sheets with enough funding to get us through our planned phase II clinical program. And we able to manufacture a product at commercial scale. So. Thank you very much, if anybody has any questions I will be upfront. Thank you.

1 comment:
Very exciting read. Thank you for posting this info.
Post a Comment